Improving the diagnostic process for breathlessness in primary care: protocol for global implementation of “Breathlessness diagnostics in a Box” (BiaB) (ID 571)
General Practitioners Research Institute (GPRI), Groningen, The Netherlands
Abstract
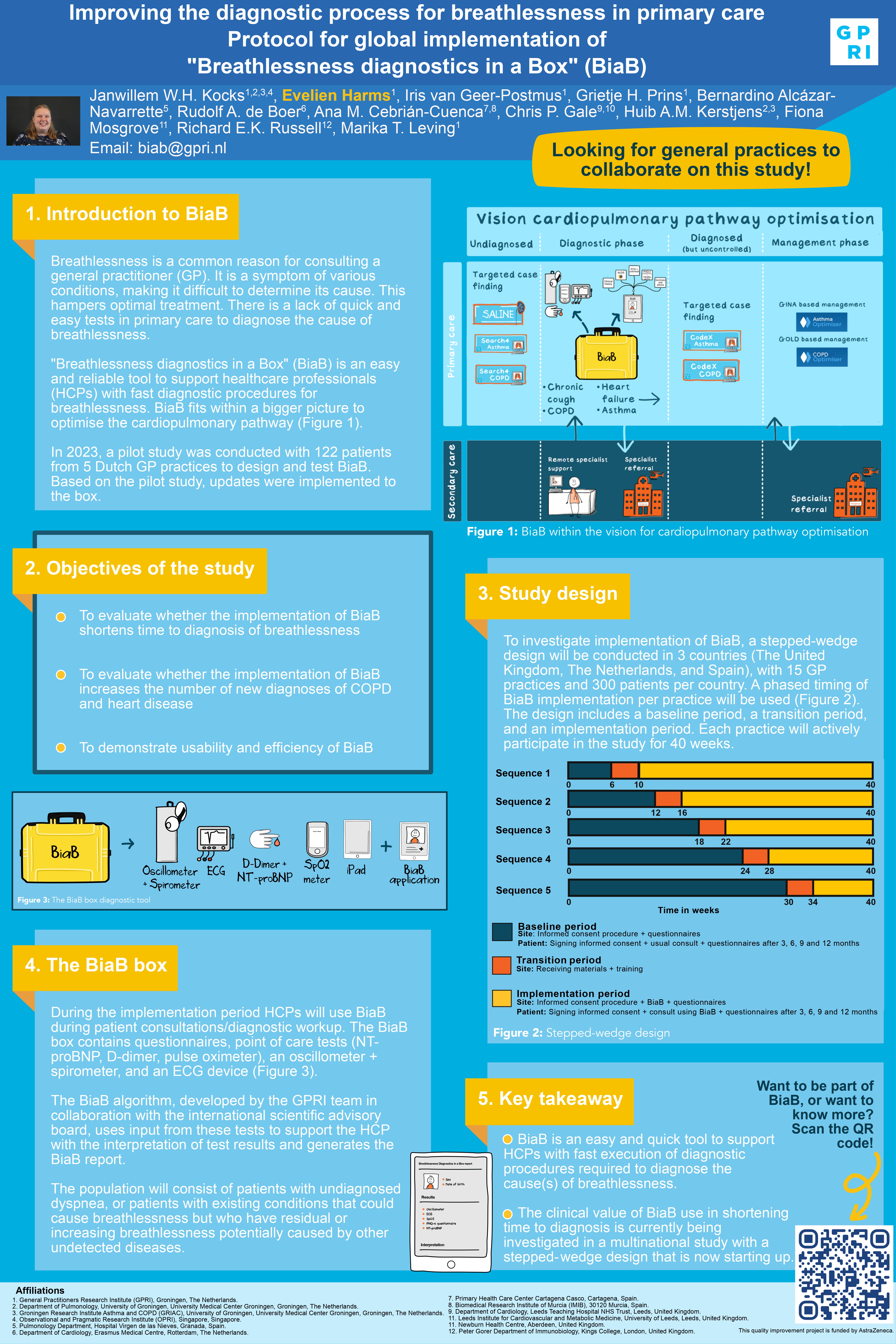
Breathlessness is a common reason for consulting a general practitioner (GP) and since it is a symptom in various conditions it is difficult to determine its cause, which hampers optimal treatment. There is a lack of quick and easy tests that can be used in primary care to diagnose the cause of breathlessness. Therefore, we have developed BiaB, “Breathlessness diagnostics in a Box”: an easy and reliable tool intended to support healthcare professionals (HCPs) with fast execution of diagnostic procedures required to diagnose the cause(s) of breathlessness. In the current study, we investigate whether implementation of BiaB in primary care will shorten the time of the diagnostic process for patients presenting with breathlessness.
To investigate implementation of BiaB, a stepped-wedge trial will be conducted in at least 3 countries, in 15 GP practices and 300 patients per country.
The study population will consist of patients with undiagnosed dyspnea and patients who have existing diseases that could cause breathlessness but have residual or increasing breathlessness that could be caused by other not yet detected diseases.
During the intervention period HCPs will use BiaB during patient consultations/diagnostic workup. The BiaB-box contains questionnaires, point of care tests (NT-proBNP, D-dimer, pulse oximeter), an oscillometer and an ECG device.
The BiaB-algorithm, developed by the GPRI team in collaboration with the international scientific advisory board, uses input from these tests to support the healthcare professional with interpretation of test results and generates the BiaB-report.
To investigate whether BiaB implementation shortens the time to diagnosis, we will evaluate the number of days between presentation with or consultation for breathlessness and a diagnosis causing breathlessness (usual care versus BiaB intervention). Secondary objectives are to demonstrate whether BiaB use increases the number of new diagnoses of COPD and cardiovascular disease as compared to usual care and to demonstrate the usability and efficiency of BiaB.
We have developed BiaB as an easy and quick tool to support HCPs with fast execution of diagnostic procedures required to diagnose the cause(s) of breathlessness. In the current study we will investigate implementation of BiaB on a large scale using a stepped-wedge study design.
Funding: This quality improvement project is funded by AstraZeneca.
Conflicts of interest: Professor Kocks reports grants, personal fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from Boehringer Ingelheim, grants and personal fees from Chiesi Pharmaceuticals, grants, personal fees and non-financial support from GSK, grants and personal fees from Novartis, grants from MundiPharma, grants from TEVA, outside the submitted work. IvG, ML, GP, EH, were employed by General Practitioners Research Institute (GPRI) at the time of the study. In the past three years (2022-2024), GPRI conducted investigator- and sponsor-initiated research funded by non-commercial organizations, academic institutes, and pharmaceutical companies (including AstraZeneca, Boehringer Ingelheim, Chiesi, Eli Lilly, GSK, Mundipharma, Novartis, Teva, and Valneva)