🏅 Conference Winning Abstract
Poor adherence in exacerbating COPD patients: magnitude and related factors at baseline in the MAGNIFY pragmatic trial (ID 346)
Observational and Pragmatic Research Institute, United Kingdom
Abstract
Aims
Commonly prescribed maintenance inhaled therapies for the treatment of COPD have been shown to reduce the risk of exacerbations. However, adherence to these therapies is often poor, compromising effectiveness and wasting public healthcare resources. Utilising technology to provide adherence support for a single dual therapy inhaler may offer a solution and is currently being investigated in the pragmatic cluster randomised trial MAGNIFY. Here we assessed MAGNIFY baseline data to understand the extent, and baseline factors associated with, poor adherence in exacerbating COPD patients.
Methods
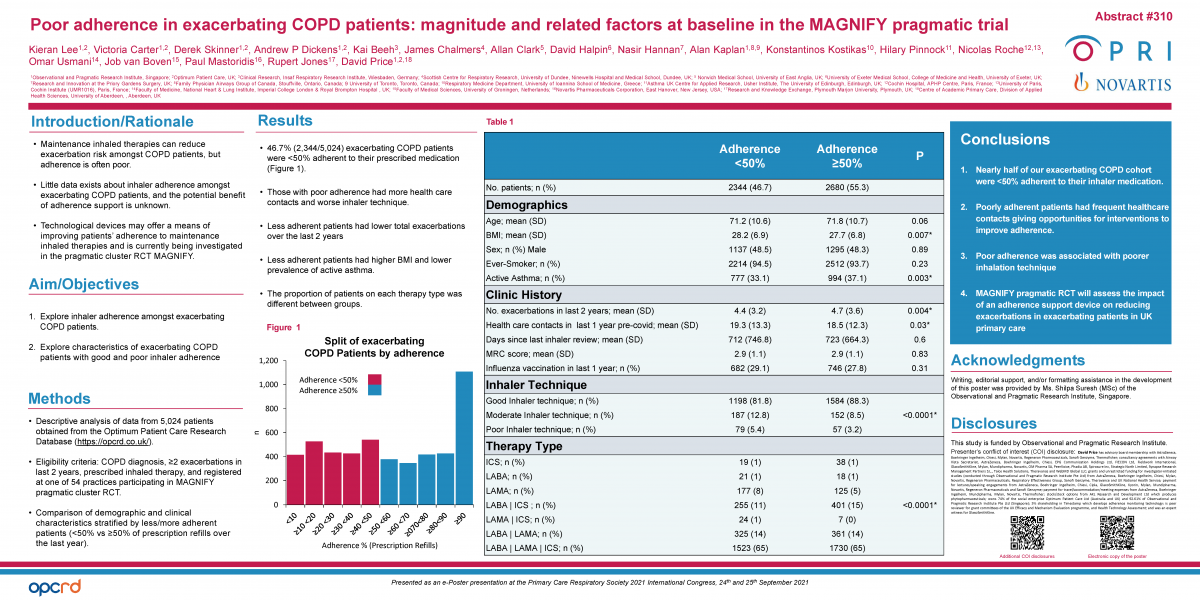
Demographic, clinic history, inhaler use, and therapy data of all COPD patients with 2 or more exacerbations over the last 2 years, on treatment and registered at one of the 54 practices participating in MAGNIFY was obtained from the Optimum Patient Care Research Database (https://opcrd.co.uk/). We compared the clinical and demographic characteristics of less adherent (<50% of prescriptions refills over the last year) and more adherent (≥50%) patients.
Results
2,344 out of 5,024 (46.7%) exacerbation-prone COPD patients had poor adherence to their prescribed medication. These patients had significantly more health care contacts and poorer inhalation technique compared to the more adherent patients. BMI, asthma, total exacerbations and therapy type were also different between groups (Table 1).
Conclusion
Nearly half of our COPD cohort at risk of exacerbations were found to be poorly adherent to their medication. Some differences were identified between less and more adherent patients, but most appeared clinically marginal and predicting adherence from our measured characteristics is not possible. Poorly adherent patients had frequent healthcare contacts giving opportunities for interventions to improve adherence. Technology support, as tested in MAGNIFY, may be a solution.
Funding: This study was co-funded by Novartis and Observation and Pragmatic Research Institute.
Conflicts of interest: Kieran Lee, Derek Skinner are employees of Observational and Pragmatic Research Institute, who co-funded the study with Novartis.
Victoria Carter is an employee of and owns shares at Observational and Pragmatic Research Institute, who co-funded the study with Novartis and Optimum Patient Care.
Kai Beeh declares personal or institutional compensations from the following in the past 5 years: Kai Beeh is a full-time employee of insaf Respiratory Research Institute. The institution has received compensation for services on advisory boards or consulting for AstraZeneca, Berlin Chemie, Boehringer, Chiesi, Cytos, GSK, Mundipharma, Novartis, Pohl Boskamp, Sanofi, Zentiva. The institution has received compensation for speaker activities in scientific meetings supported by AstraZeneca, Berlin Chemie, Boehringer, ERT, GSK, Novartis, Pfizer, Pohl Boskamp, Sanofi, Takeda. The institution has further received compensation for design and performance of clinical trials from AstraZeneca, Boehringer, GSK, Infinity, Mundipharma, Novartis, Parexel, Pearl Therapeutics, Pfizer, Revotar, Teva, Sterna, and Zentiva
James Chalmers has received research grants or consultancy fees from Astrazeneca, Boehringer Ingelheim, Gilead Sciences, Glaxosmithkline, Grifols, Insmed and Zambon.
Allan Clark reports no conflict of interest.
David Halpin has received personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Pfizer and Sanofi, and non-financial support from Boehringer Ingelheim and Novartis.
Nasir Hannan is Director of MD innovation LTD, Director of MINT medial Service LTS and executive member of the Bedfordshire and Hertfordshire LMC
Alan Kaplan is a member of the advisory board of, or speakers bureau for, AstraZeneca, Behring, Boehringer Ingelheim, Covis, Grifols, GlaxoSmithKline, Merck Frosst, Novo Nordisk, Novartis, Pfizer, Purdue, Sanofi, Teva, and Trudel.
Konstantinos Kostikas was an employee and shareholder of Novartis Pharma AG until 31.10.2018. He has received honoraria for presentations and consultancy fees from AstraZeneca, Boehringer Ingelheim, Chiesi, ELPEN, GSK, Menarini, Novartis and Sanofi. His department has received funding and grants from AstraZeneca, Boehringer Ingelheim, Chiesi, Innovis, ELPEN, GSK, Menarini, Novartis and NuvoAir. He is a member of the GOLD Assembly.
Hilary Pinnock reports no conflict of interest.
Nicolas Roche reports grants and personal fees from Boehringer Ingelheim, Novartis, and Pfizer, and personal fees from Teva, GSK, AstraZeneca, Chiesi, Sanofi, and Zambon.
Omar Usmani reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi and GlaxoSmithKline. Personal fees from Napp, Mundi Pharma, Sandoz,and Takeda, Cipla, Covis, Novartis, Mereo BioPharma, Orion, Menarini, Deva, Roche, Trudel UCB, and grants from Edmond pharma outside the submitted work.
Job van Boven has received consultancy fees, honorarium and research funding from AstraZeneca, Boehringer Ingelheim, Chiesi, Menarini, Novartis, Pill Connect, Teva and Trudell Medical to consult, give lectures, provide advice and conduct independent research, all paid to his institution.
Paul Mastoridis is an employee of Novartis and holds stock in Novartis Pharma AG., who co-funded the study with Observational and Pragmatic Research Institute
Rupert Jones declares grants from Astra Zeneca, Glaxo Smith Kline, Novartis and Teva and personal fees for consultancy, speakers fees or travel support from Astra Zeneca, Boehringer Ingelheim, Glaxo Smith Kline, Novartis and OPRI.
David Price has board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Thermofisher; consultancy agreements with Airway Vista Secretariat, AstraZeneca, Boehringer Ingelheim, Chiesi, EPG Communication Holdings Ltd, FIECON Ltd, Fieldwork International, GlaxoSmithKline, Mylan, Mundipharma, Novartis, OM Pharma SA, PeerVoice, Phadia AB, Spirosure Inc, Strategic North Limited, Synapse Research Management Partners S.L., Talos Health Solutions, Theravance and WebMD Global LLC; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Theravance and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, Thermofisher; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline.